Abstract
Introduction:Oral squamous cell carcinoma (OSCC) is the most common cancer of the head and neck area, and the incidence remains high.Despite advances in diagnosis and treatment, the poor prognosis of OSCC is characterized by a high rate of local recurrence and the overall five-year survival rate remains at approximately 50%. Therefore, the mechanisms underlying the development of OSCC still need to be clarified. Patients with cancer tend to develop a hypercoagulable state which predisposes them to thromboembolic events. Cancer increases the risk of venous thrombosis several fold with varying degree of relative risks (range 4-7). A recent study has reported that microparticles (MPs) increased procoagulant activity (PCA) in OSCC. MPs are small membrane vesicles of 0.1-1 µm containing negatively charged, procoagulant phosphatidylserine (PS), which plays an important role in thrombosis. The definitive role of PS in the hypercoagulable state in patients with OSCC remains unclear. Our objectives were to measure the PS exposure on MPs, blood cells, and endothelium, and to evaluate their PCA in different stages of OSCC.
Methods: OSCC patients (n = 57) and healthy controls (n = 26) were included in our study. Blood samples were obtained from controls and OSCC patients within 1 day before surgery and 2-week after surgery. Human umbilical vein endothelial cells (HUVECs) were incubated in growth media containing 20% of pool serum obtained from either OSCC patients or healthy donors at room temperature for 24 h, respectively. Exposed PS was analyzed with flow cytometry and confocal microscopy. Lactadherin was used to quantify PS exposure on MPs and their original cells. PCA of MPs and these cells was evaluated using clotting time, purified coagulation complex, and fibrin formation assays. Meanwhile, the inflammation-related cytokines were detected by enzyme-linked immunosorbent assay.
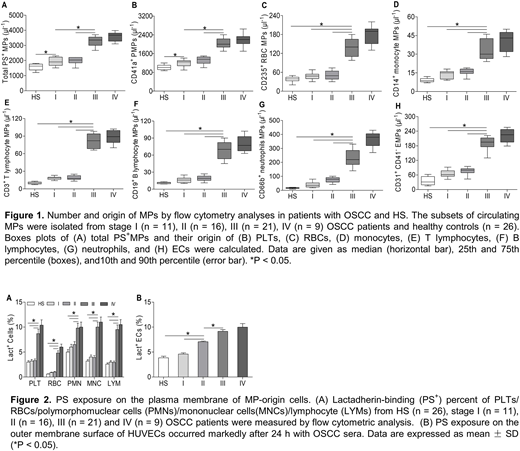
Results: Using flow cytometry, plasma levels of PS+ blood cells and MPs in OSCC patients with stage III/IV were significantly higher than those in stage I/II patients or healthy controls (all P < 0.05). However, we only found a significant difference between stage I or II and controls (P < 0.05) in total PS+ MPs and PMPs. Similarly, we found that the endothelial cells (ECs) treated with OSCC serum in vitro exposed more PS than those with healthy serum. Moreover, in OSCC patients with stage IV, MPs primarily originated from platelets (53.9 ± 3.2%) followed by leukocytes (21.8 ± 2.1%, including MPs from neutrophils, monocytes and lymphocytes), erythrocytes (6.3 ± 0.6%) and ECs (6.9 ± 0.8%). Additionally, PS+ blood cells, MPs and OSCC serum-cultured ECs markedly promoted shortened coagulation time and significantly increased FXa/thrombin/fibrin generation in stage IV or III OSCC patients compared with controls (all P < 0.05). Interestingly, confocal imaging of MPs or OSCC serum-treated ECs showed binding sites for FVa and FXa to form prothrombinase. Furthermore, blockade of PS on MPs/blood cells/ECs with lactadherin inhibited PCA by approximately 80%. Most importantly, we found treatment with radical resection significantly reduced the amount of PS+ blood cells, ECs and MPs, and prolonged the clotting times of those cells and MPs compared with presurgery patients. Lastly, the correlation analysis revealed that the plasma levels of interleukin 6, interleukin 8 and tumor necrosis factor α were positively correlated with the levels of total PS+ MPs and PS+ platelets in OSCC patients.
Conclusions: Our results suggested that PS+ blood cells and MPs play a prominent role in inducing the hypercoagulable and prothrombotic state especially in advanced OSCC. Interestingly, we found treatment with radical resection could attenuate this effect. Moreover, the released inflammatory cytokines may contribute to PS exposure on platelets and MPs and the increased procoagulant activity in patients with OSCC. Notably, our findings also show that PS provides binding sites for FXa and prothrombinase complexes and promotes thrombin formation. Therefore, directly targeting FXa and prothrombinase complexes might decrease thrombotic risk OSCC patients. This study shows that future research should focus on the application of PS inhibitors as a novel therapeutic strategy in OSCC patients when coagulation is abnormally enhanced.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.